IFPMA statement on behalf of the pharmaceutical industry at the Convention on Biological Diversity (CBD) COP 15 meeting regarding Digital Sequence Information.
Read moreDuring the World Health Organization (WHO) Third Meeting of the Intergovernmental Negotiating Body, IFPMA delivered four messages.
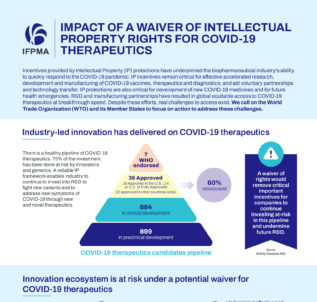
Read moreAs WTO Member States continue to discuss an extension of a waiver of intellectual property (IP) rights on COVID-19 therapeutics, latest evidence and data published today explains what the consequences of such a waiver would have on industry’s ability to fight the COVID-19 pandemic.
Read more
New annual research conducted by Ipsos UK, shows that the research-based pharmaceutical industry has garnered a positive reputation, with its pandemic response and the impact of its innovations seen as key strengths by the general public.
Read moreThe Japanese Pharmaceutical Manufacturers Association (JPMA) and the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) jointly submitted the “Proposals from the Pharmaceutical Industry for the G7 Hiroshima Summit Health Agenda” to the Prime Minister, Minister of Health, Labor and Welfare, Minister of Finance, and Minister of Foreign Affairs.
Read moreThe pace of resistance to existing antibiotics is outpacing the rate at which new ones can reach the market and be used against difficult-to-treat infections.
Read more
Responding to the impact of the Nagoya Protocol on Access and Benefit Sharing
Read more
This World Diabetes Day (WDD), IFPMA and its members are renewing our commitment to take proactive steps to improve access to diabetes care and make progress toward the achievement of SDG target 3.4.
Read more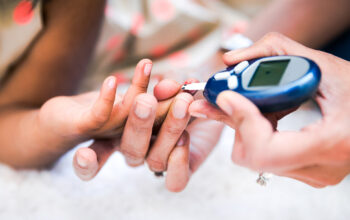
The International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) was a founding member of ACT-A when it was launched in April 2020. We welcomed the vision of the ACT-A to involve the private sector, including the innovative pharmaceutical industry, to join this unique global collaboration set up to accelerate development, production, and equitable access to...
Read moreIFPMA welcomes the opportunity to make this statement and to share our Covid-19 Lessons Learned, which highlights that swift pathogen surveillance and sharing, an enabling innovation ecosystem, and regulatory agility must be preserved to ensure our collective ability to fight against unknown diseases.
Read moreIFPMA appreciates the opportunity to make this statement. The ongoing fight against COVID-19 has shown that those with chronic conditions and co-morbidities are often the most vulnerable and have suffered the most throughout the pandemic.
Read moreBiopharmaceutical industry’s considerations on key elements for an effective multilateral Pandemic Prevention, Preparedness, and Response (PPR) instrument.
Read more