On 5 August 2025, IFPMA, together with Health Care Without Harm and the Global Self-Care Federation, published a joint statement on the global plastics treaty under negotation at the continued fifth session of the Intergovernmental Negotiating Committee (INC-5.2) in Geneva.
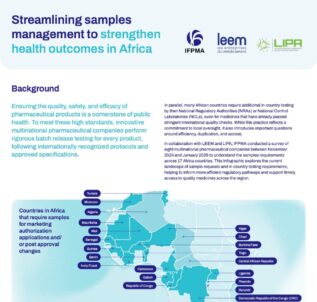
Read moreEnsuring the quality, safety, and efficacy of pharmaceutical products is a cornerstone of public health. To meet these high standards, innovative multinational pharmaceutical companies perform rigorous batch release testing for every product, following internationally recognized protocols and approved specifications. However, countries may require additional in-country testing by their National Regulatory Authorities (NRAs) or National Control...
Read more
On 11 July 2025 in New York at the UN Headquarters, IFPMA delivered a statement at the High-Level Dialogue on the Determinants of Health. Innovation in public health is essential to addressing the social, economic, and environmental determinants of health. IFPMA welcomes this dialogue and underscores the importance of collaboration to address complex health challenges,...
Read moreReflections on the draft Political Declaration for the UN High Level Meeting on the Prevention and Control of NCDs and the Promotion of Mental Health and Well-being This advocacy brief outlines three areas where industry seeks more explicit commitments from governments: Leverage health innovation Strengthen prevention and early action for lifelong health Address multimorbidity through...
Read more
During the OECD Global Public Procurement Forum, leaders will discuss how public procurement can be used to unlock social, environmental and economic benefits. Sustainable procurement can be an opportunity for collaboration between healthcare systems and industry to get this balance right. However, for this approach to be successful, it must be carefully implemented. Without a thoughtful design, procurement policies can inadvertently introduce requirements that disrupt supply chains, limit product availability, and put the brakes on future innovation. In this blog, IFPMA shares a set of core principles that can guide policy design on sustainable procurement.
Read more
On 26 June 2025, six leading international organizations representing patients, physicians, pharmacists, nurses, hospitals, and the pharmaceutical industry have adopted the first joint ethical principle in the healthcare industry on the responsible use of health data and technology, including artificial intelligence.
Read more
The International Consensus Framework for Ethical Collaboration in Health is the only global platform of its kind, routinely convening diverse health stakeholders in support of high-quality patient care. First established in 2014, the Consensus Framework was revised in 2024 to coincide with its tenth anniversary and adopted in 2025 to include a new principle, on responsible use of health data and technology. This reflects a growing importance of digital health and artificial intelligence, and a need for ethics to evolve alongside innovation.
Read more
On 25 June 2025, Gavi, the Vaccine Alliance, is co-hosting the Global Summit: Health & Prosperity through Immunization in support of its next five-year strategic period, in partnership with the European Union and the Gates Foundation.
Read moreMedicines and vaccines have transformed the way we prevent, treat, and cure diseases. Bringing these to people around the world requires more than scientific breakthroughs – it demands collaboration across the entire innovation and access pathway. From discovery and development to regulatory approval, supply, procurement and local delivery, each step plays a critical role in...
Read more
Ahead of the G7 Leaders’ Summit in Kananaskis from 15 to 17 2025, the global pharmaceutical industry, represented by the International Federation of Pharmaceutical Manufacturers & Associations (IFPMA) and Innovative Medicines Canada (IMC), are calling on G7 governments to place health and innovation at the top of their agenda.
Read moreAhead of the G7 Leaders’ Summit, IFPMA and Innovative Medicines Canada (IMC) reaffirm the innovative pharmaceutical industry’s commitment to partnering with G7 governments to improve patient outcomes, strengthen health systems, boost economic growth, and support national security.
Read more
Dr. David Reddy, Director General, IFPMA, and Dr. Bettina Hamelin, President, IMC, jointly advocate for health innovation at the 2025 G7 Summit in Kananaskis (15-17 June). They call G7 leaders to place health and life-sciences innovation at the heart of the G7 agenda, emphasizing three areas of focus where the pharmaceutical industry can drive impact.
Read more