
Viruses don’t stand still. And neither do we. It’s been almost two years since the administration of the first COVID-19 vaccine in December 2020 and as of September 2022, more than two thirds of people worldwide had received at least one COVID-19 vaccine dose.
Read moreIFPMA is delighted to be able to join this SEARO Regional Committee meeting and appreciates the opportunity to make this statement
Read moreIFPMA welcomes the opportunity to comment on the WHO AFRO region’s strategy for health security and emergencies. The innovative biopharmaceutical industry is keen to learn lessons from the COVID-19 pandemic and to work with others to ensure we are much better prepared for the next health emergency. We are not surprised by the assessment from...
Read morePrepared for IFPMA, Airfinity have authored a report detailing the COVID-19 pandemic in Africa, with a key focus on vaccinations.
Read more
This infographic provides an overview of the status of COVID-19 vaccines in Africa, outlining issues of inequitable distribution on the continent, factors contributing to this, and policy recommendations to ensure equitable access to vaccines in future.
Read more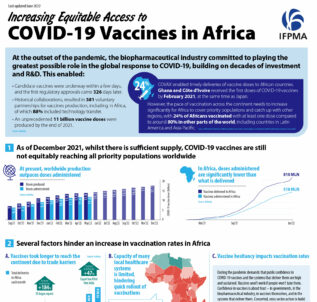
We congratulate you on the early progress made on this important and complex work. We will share written comments in due course. At a high level, we welcome the recognition that the development of new medicines rests on the protection of intellectual property rights; that our ability to develop medical countermeasures relies on unhindered and...
Read moreThe innovative biopharmaceutical industry, having developed COVID-19 vaccines and treatments at record speed and in historic quantities, proposes to create a collaborative solution for more equitable rollout of vaccines, treatments and diagnostics for future pandemics. In particular, the innovative biopharmaceutical industry is willing to reserve an allocation of real-time production of vaccines, treatments and diagnostics...
Read moreThe biopharmaceutical industry launched today the “Berlin Declaration – biopharmaceutical industry vision for equitable access in pandemics” and presents global leaders with a proposal that could help ensure the supply of pandemic vaccines, treatments and diagnostics are delivered as early as possible in future pandemics to those who need them most.
Read moreThe task of taking quality care to those living with cancer grows more difficult with the exploding number of new cases. A new initiative pools together expertise to widen access to timely and effective treatment.
Read more
Artificial Intelligence (AI) has the potential to bring about positive transformations in science and society, including in healthcare. As a powerful enabler of progress and innovation, AI can increase individual well-being and contribute to the common good. However, while offering tremendous opportunities, relatively new AI technology could also raise concerns relating to potential misuse and inadequate accountability, as well as systemic risks inherent to algorithmic bias and discrimination.
Read more
In the wake of COVID-19, lower-resourced settings need sustainable healthcare more than ever to build back better. Promising solutions are at hand.
Read moreThis policy briefing summarizes trends in reported experiences (from primary and secondary research) in the use of regulatory agilities applied to quality observed since the start of the pandemic, some reported challenges to their implementation and forward-looking recommendations, whether to prepare for the next pandemic or strengthen standard normative processes to accelerate patient access to safe and effective medicines and vaccines.
Read more