
On 5 May 2025, IFPMA, EFPIA, and EUCOPE issued a statement supporting the World Health Assembly Resolution and Global Action Plan on Rare Diseases, in line with a call to action by a coalition of civil society organizations.
Read moreOn 2 May 2025 in New York, IFPMA delivered a statement at the UN Multi-stakeholder Hearing on the Prevention and Control of Noncommunicable Diseases (NCDs) and the Promotion of Mental Health and Well-being.
Read moreChronic diseases are on the rise. Vaccines can help reduce the impact on people, health systems and society. This year’s UN High-level Meeting on NCDs and Mental Health provides an opportunity to maximise vaccine benefits. Non-communicable diseases (NCDs), such as cancer, cardiovascular disease, chronic respiratory diseases and diabetes, are the leading cause of ill health...
Read more
On 17 April 2025, IFPMA and IPASA submitted an open letter to G20 Ministers in support of the advancement of the G20 Health Agenda.
Read moreOn the conclusion of the 13th resumed meeting of the Intergovernmental Negotiating Body in Geneva, IFPMA delivered a statement.
Read moreOn 7 April in Geneva, IFPMA delivered a statement at the 13th resumed meeting of the INB to agree a WHO instrument for pandemic prevention, preparedness, and response.
Read moreThe Biopharmaceutical CEO Roundtable (BCR), which represents the world’s leading biopharmaceutical companies, met in London. 04 APRIL 2025, London – This week, the Biopharmaceutical CEO Roundtable (BCR), which represents the world’s leading biopharmaceutical companies, met with the UK Prime Minister, senior UK Cabinet Ministers and key officials to discuss strengthening global health policies and sustaining...
Read more
Strong regulatory frameworks provide a predictable and efficient pathway for scientific advancements to translate into real-world impact, ultimately leading to better health outcomes for patients worldwide. National Regulatory Authorities (NRAs) play a vital role in ensuring that patients receive safe and effective medical products. Their collaboration and constant dialogue with pharmaceutical companies at every stage...
Read more
Non-communicable diseases (NCDs) including cancer, diabetes, cardiovascular disease, lung disease, mental health, and neurological disorders are responsible for 75% of deaths worldwide. New research demonstrates that investing an additional 1% of GDP in public healthcare spending, where at least 40% is aimed at preventing and treating NCDs, could save close to 5 million lives each...
Read moreAhead of the 14th WTO Ministerial Conference (MC14), trade associations representing the pharmaceutical industry provide a perspective on how WTO Members can a roadmap to strengthen global health through meaningful trade policies.
Read more
In new research commissioned by IFPMA, Airfinity has determined that an additional investment of 1% of GDP toward domestic general government health expenditure in low- and middle-income countries (LMICs) would amount to a US$ 310 billion additional investment, representing a 33% increase in current spending. This research is a response to the UHC 2030 action agenda...
Read more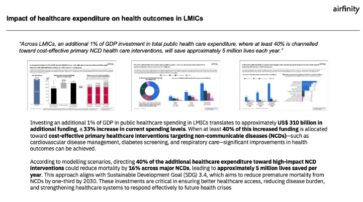
Non-communicable diseases (NCDs), such as diabetes, cardiovascular, renal and metabolic diseases, cancer, chronic respiratory diseases, mental health and neurological diseases, are one of the greatest global health and economic threats we face in our lifetime. Since 2000, global deaths due to NCDs have increased rapidly, even as deaths due to communicable diseases have declined. The...
Read more