Is an extension of the TRIPS waiver needed for COVID-19 tools?

Evidence shows that progress made in sharing IP through voluntary agreements, combined with tiered-pricing and not-for-profit arrangements for low- and middle-income countries has led to production of COVID-19 therapeutics exceeding demand for all disease severity levels and in all patient settings.
Efforts must now focus on ensuring these products reach those in need.
Last week, we saw yet another powerful example of the spirit of collaboration and partnership that has supported the global response against the COVID-19 pandemic. The Medicines Patent Pool (MPP) signed another voluntary license agreement with Japanese pharmaceutical company, Shionogi, to enable generic companies to produce its COVID-19 antiviral treatment pill candidate, provided it gets regulatory approval.
This voluntary license agreement adds to the over 140 collaborations already established by innovative pharmaceutical companies with partners across the world. In the first year of the pandemic alone, companies developing COVID-19 treatments entered into over 40 collaborations to expand manufacturing capacity. Many – like Shionogi, but also Gilead – did so even before regulatory authorities granted full regulatory approval, reflecting commitments to broad patient access and allowing for time to establish a robust framework with partners that would allow them the time and knowledge necessary to produce safe, quality and effective treatments.
The number of collaborations has more than tripled since then. Today, 92% of these collaborations involve technology transfer, which proves that the voluntary approach has been widely embraced as a solution scaling up manufacturing of COVID-19 treatments. Voluntary licenses are not just a matter of simply licensing intellectual property and walking away. To make the collaboration really work, and ultimately result in the treatments reaching the people who need them, companies work hand in hand sharing technology and know-how, building internal capacity, solving problems as they emerge and celebrating successes.
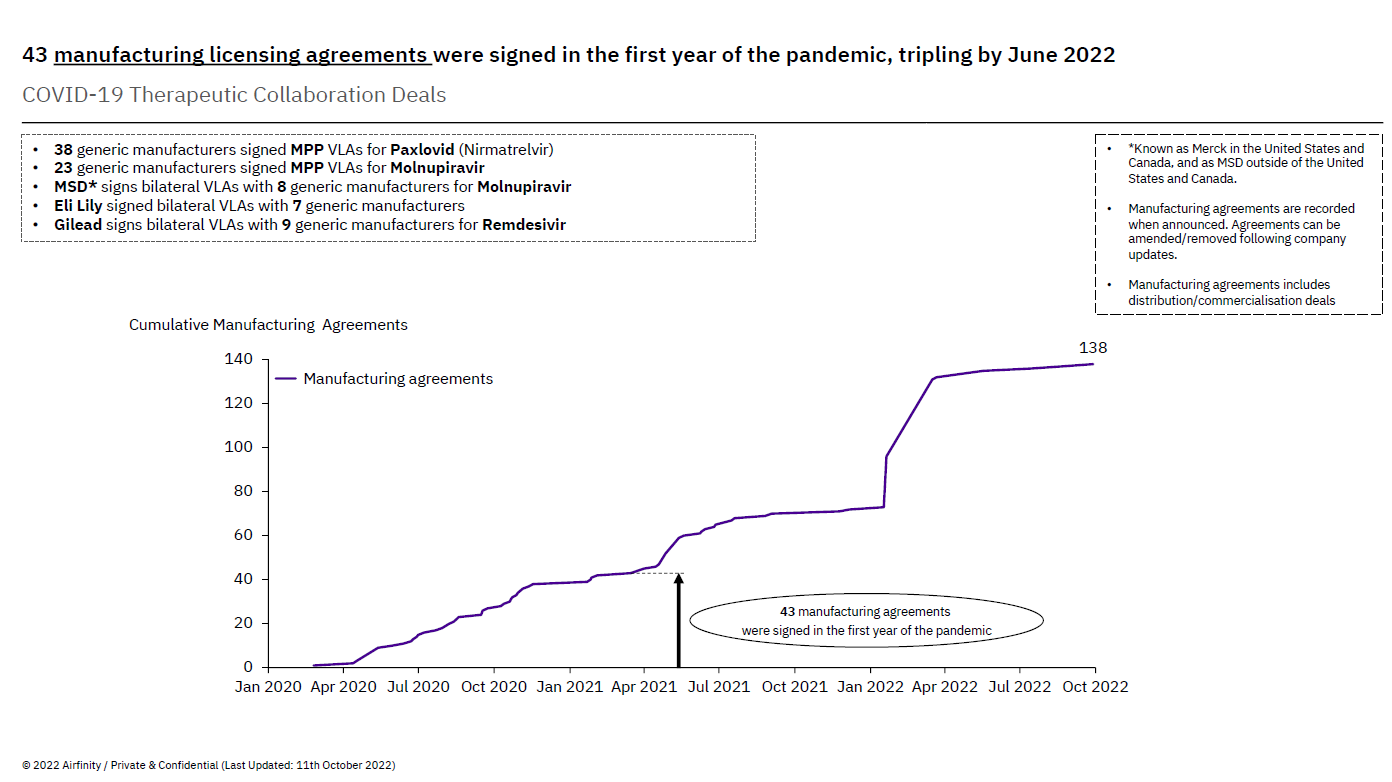
Voluntary licenses on therapeutics are already in place to support manufacturing scale up without needing a TRIPS waiver
Biopharmaceutical companies have acted decisively early on by putting equitable access to therapeutics at the top of their priorities. Far from being a hindrance to ramping up global supply, IP rights have provided companies with legal certainty for their discoveries, as well as created trust to engage with other partners for such deals. In addition to voluntary licensing, pharmaceutical companies have also implemented multiple strategies to enhance access, including tiered pricing.
The number of existing voluntary license agreements (bilaterally and through Medicines Patent Pool) is a testament to this. For example, MSD, which makes molnupiravir (Lagevrio), and Pfizer, which makes nirmatrelvir/ritonavir (Paxlovid), have struck voluntary licensing agreements with 70 generic manufacturers in Asia, Africa, and Latin America. Gilead, which makes remdesivir (Veklury), has issued voluntary licenses to nine generic manufacturers, covering 127 countries.
Supply for treatments exceed demand and a WTO TRIPS waiver would not result in more access to therapeutics
Developing countries have faced unjust scarcity and hardship in obtaining COVID-19 tools, which the biopharmaceutical industry has been vocal about as well. But extending the TRIPS waiver would do nothing to address the real barriers to access.
Even though it takes time to set the agreements up, already, data indicates that currently, production exceeds demand for treatments for all disease severity and patient setting.
Partnerships with multilateral organizations and over 140 voluntary licensing partnerships made it possible to ramp up production of therapeutics and, combined with tiered pricing and not-for-profit arrangements for low- and middle-income countries, mean that even in resources-limited settings, governments have effective tools to give their population access to the key therapeutics.
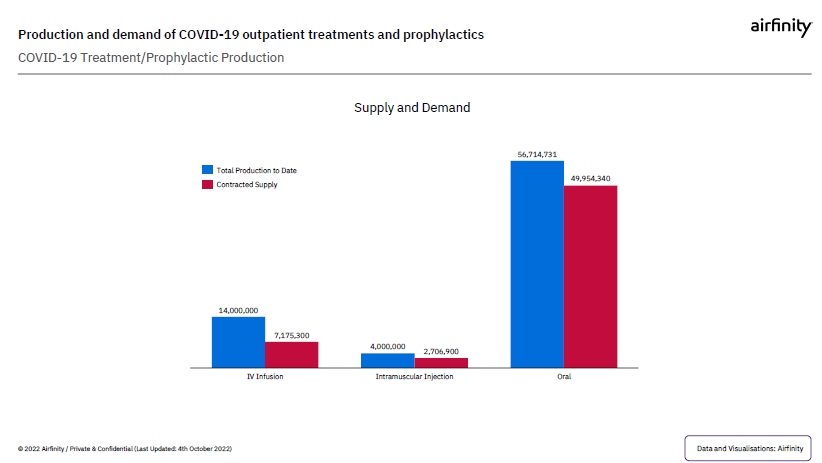
What’s more, in recent months, Airfinity has revised demand forecasts downward and there is no evidence to suggest that supply is constrained relative to actual demand.
Unintended consequences of a WTO TRIPS waiver will undermine generic manufacturers who have signed agreements, overburden regulatory systems without any upside for patients
The TRIPS waiver extension that is being discussed will have unintended consequences and will undermine the business of the generic manufacturers who have signed voluntary agreements. It may also, even more worryingly, impact the very people that need help.
In the first instance, a waiver may create a counterproductive proliferation of manufacturers, who would operate outside of voluntary agreements that foster trust and knowledge-sharing. Basically, it would put the legal basis for these existing collaborations at a disadvantage and potentially jeopardize them, including those under the MPP agreements.
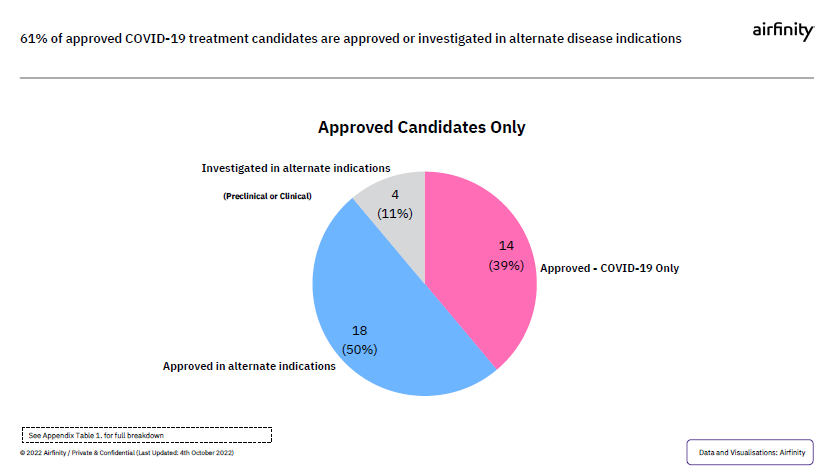
Beyond COVID-19, if the waiver is approved, it could potentially impact innovation to treat other diseases. Currently of the 2% of treatments that have made it through the pipeline and are approved for COVID-19, more than half are either approved or could have other uses. As hopefully more innovations get approved, the waiver could potentially undermine the legal certainly industry needs to continue innovating, ultimately hurting patients worldwide.
Rather than a TRIPS waiver, we need more focus on test and treat
While innovator pharmaceutical companies and their hundreds plus licensees continue to increase manufacturing output to supply the world, there is a concern that local systems do not have the absorption capacity for the new therapeutics to be most impactful in curbing the effect of the COVID-19 pandemic. More efforts are needed to ensure that eligible patients can access the COVID-19 therapeutics that are being produced, at the right time. Low levels of testing, as well as insufficient capacity to distribute and administer treatments, has left uptake of therapeutics low, and put millions of doses at risk of going unused and being destroyed. After peaking during the Omicron wave, the frequency of testing for COVID-19 has fallen globally.
… And we need more innovation, unhampered by uncertainty of an unnecessary TRIPS waiver
Our conclusion is that, rather than a TRIPS waiver and the uncertainty that comes with it for no apparent improvement in equity, the innovation pipeline needs to be encouraged. The IP framework has been a key enabler for an unprecedented pace of R&D and manufacturing scale-up. It’s what’s given confidence to public and private researchers to investigate thousands of molecules that could work against COVID-19, supporting breakthroughs in our pandemic response. As the virus evolves, so must further research, supported by strong IP protections.
As WTO Member States engage in discussions around an extension of a TRIPS waiver to COVID-19 therapeutics and diagnostics, the innovative pharmaceutical industry calls on researchers from their labs join us in urging policymakers to consider the data and evidence-base that highlights not only that such an extension is not needed, but how it would in fact have a consequential impact on modern health innovation and patients.
Author






