New principles on incentivizing antibiotic R&D
Antibiotics have transformed healthcare. But today, the ubiquitous use of these live-saving drugs has led to the development of antibiotic-resistant bacteria. Once-treatable diseases like common infections of the bloodstream, urinary or respiratory tracts are becoming more difficult to treat using these tools. Combating drug resistance means facing a silent pandemic that could dwarf the impact COVID-19 has had on health systems and economies around the world.
The threat of antimicrobial resistance is not new – it has been on the agenda of politicians for several years already, but there has been little action to address the enduring economic puzzle we see at play in antibiotic R&D.
The current pipeline is widely recognized as insufficient. For example, there are currently approximately 40 antibiotics in clinical development, of which only a handful are considered novel, and only one new class of antibiotics has been launched in recent decades. Another 21 non-traditional antibacterial products and 12 antifungal agents are currently in clinical development. Because bacteria continually develop resistance to our existing antibiotics, we will always need a robust pipeline of new ones – so it is critical to have sustained investment to keep pace with growing resistance. More investment is also needed into vaccines and diagnostics to help prevent infections and support appropriate use of antibiotics.
Now, more than ever, we need to accelerate the creation of a vibrant and sustainable innovation ecosystem to support R&D for new antibiotics and other antimicrobials addressing pathogens prioritized by leading public health bodies.
2025 will mark ten years since the adoption of the WHO Global Action Plan (GAP) on AMR: we need to accelerate progress to address the ‘sustainable economic case’ objective of the GAP before then.
That’s why we are calling on countries to take steps now to deliver a clear implementation roadmap by the end of 2021 that addresses:
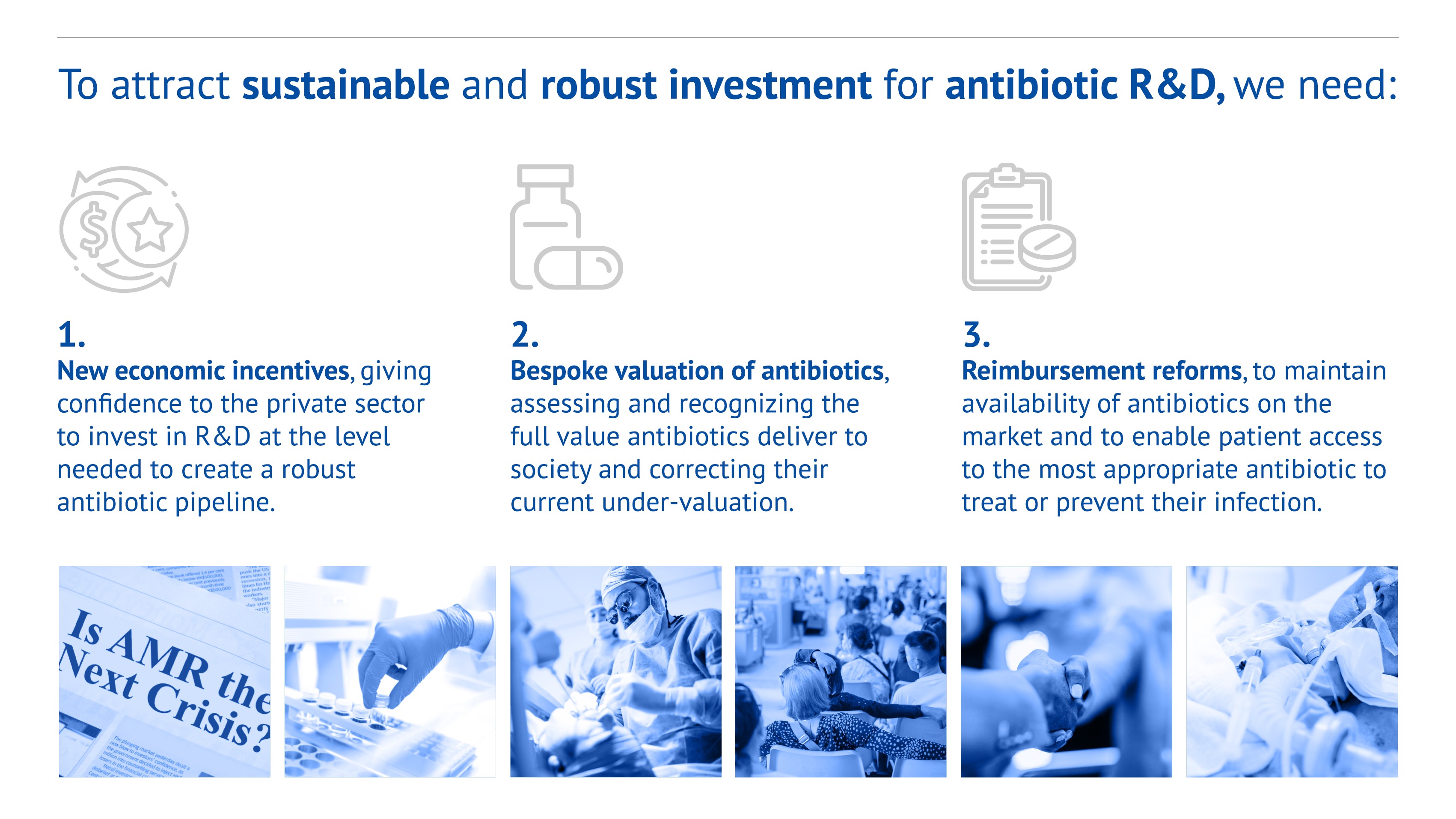
- New economic incentives, giving confidence to the private sector to invest in R&D at the level needed to create a robust antibiotic pipeline.
- Bespoke valuation of antibiotics, assessing and recognizing the full value antibiotics deliver to society and correcting their current under-valuation.
- Reimbursement reforms, to maintain availability of antibiotics on the market and to enable patient access to the most appropriate antibiotic to treat or prevent their infection.
The solutions to these challenges will look different in different countries – there is no ‘one size fits all’ – but this supportive policy framework is necessary to drive long term investments in innovative antibiotics, throughout the discovery, development, and product lifecycle.
Author