Our perspectives
in one place
Topic
Selected filters
ClearNews
See allTogether for lifelong health
UN HLM on NCDs and Mental Health: Joint statement on the importance of embedding life-course immunization and integrated multimorbidity approaches in NCD prevention and care On the occasion of the UN General Assembly’s Fourth High-Level Meeting on NCDs and Mental Health, the International Federation on Ageing (IFA), the Global Allergy & Airways Patient Platform (GAAPP),...
Read moreUN High-Level Meeting (HLM) on the Prevention and Control of Non-communicable Diseases (NCDs) and the Promotion of Mental Health
On 25 September 2025, IFPMA delivered a statement at the UN High-Level Meeting on NCDs and Mental Health in New York.
Read moreUN Political Declaration can accelerate action on non-communicable diseases (NCDs) and mental health
On 25 September 2025, governments discussed a Political Declaration aimed at accelerating global efforts to prevent and control NCDs and promote mental health, including through a one-third reduction of premature mortality from these conditions by 2030. Commenting on the High-Level Meeting on NCDs and Mental Health, Dr David Reddy, Director General of IFPMA, said: “The...
Read moreStatement on PABS system at the second meeting of the open-ended Intergovernmental Working Group (IGWG 2) on the WHO Pandemic Agreement
On 15 September 2025 in Geneva, IFPMA delivered a statement at at the second meeting of the open-ended Intergovernmental Working Group (IGWG 2) on the WHO Pandemic Agreement.
Read moreStatement on the publication of the 24th WHO Model List of Essential Medicines
Responding to the publication of the updated WHO Model List of Essential Medicines (EML), IFPMA Director General, Dr. David Reddy said: “The inclusion of innovative medicines on the updated EML highlights how scientific advances are transforming how we prevent, treat and cure disease, and reinforces the importance of ensuring patients everywhere can benefit from them. ...
Read moreAMATA statement at 75th WHO AFRO: Progress report on the regional strategy on regulation of medical products in African region
On 27 August 2025, the African Medicines Agency Treaty Alliance submitted a statement on agenda item 16.8: Progress report on the regional strategy on regulation of medical products in African region, 2016-2025 at the 75th Session of the WHO Regional Committee for Africa. The African Medicines Agency Treaty Alliance (AMATA) commends the progress made under...
Read morePublications
See allRevitalizing the antibiotic pipeline by implementing new R&D pull incentives
With the 2024 UN High-Level Meeting on antimicrobial resistance (AMR) and its political declaration, together with the biennial AMR Ministerial Meeting, the world has set an ambitious agenda to tackle AMR. The next four years offer renewed political momentum to deliver progress ahead of the next UN High-Level Meeting on AMR in 2029. A central priority is creating the necessary policy frameworks that stimulate R&D investment. This brief identifies the key barriers to antibiotic R&D and the policy measures needed to address them. Sustainable, effective pull incentives are urgently required to ensure a reliable pipeline of new antibiotics for patients and health systems. We set out how these incentives should be designed and implemented, guided by a set of core principles to maximize their impact.
Read more
Reimagining our response to non-communicable diseases (NCDs) and mental health
Reflections on the draft Political Declaration for the UN High Level Meeting on the Prevention and Control of NCDs and the Promotion of Mental Health and Well-being This advocacy brief outlines three areas where industry seeks more explicit commitments from governments: Leverage health innovation Strengthen prevention and early action for lifelong health Address multimorbidity through...
Read more
International consensus framework for ethical collaboration in health
The International Consensus Framework for Ethical Collaboration in Health is the only global platform of its kind, routinely convening diverse health stakeholders in support of high-quality patient care. First established in 2014, the Consensus Framework was revised in 2024 to coincide with its tenth anniversary and adopted in 2025 to include a new principle, on responsible use of health data and technology. This reflects a growing importance of digital health and artificial intelligence, and a need for ethics to evolve alongside innovation.
Read more
Expert insights
See all
Getting procurement right: sustainable and secure supply chains that support access to medicines
Read more
G7 Canada is an opportunity to unlock the potential of life sciences
Read more
Global resilience and national security starts with preventing ill health
Read moreResources
See allStreamlining samples management to strengthen health outcomes in Africa
Ensuring the quality, safety, and efficacy of pharmaceutical products is a cornerstone of public health. To meet these high standards, innovative multinational pharmaceutical companies perform rigorous batch release testing for every product, following internationally recognized protocols and approved specifications. However, countries may require additional in-country testing by their National Regulatory Authorities (NRAs) or National Control...
Read more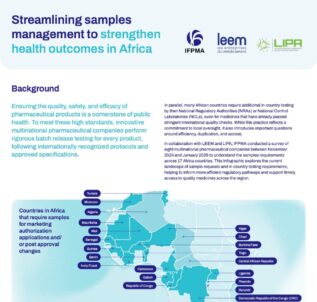
Our Ethos in Action – Decision-Making Framework Toolkit
IFPMA has developed a Five-Phase Decision-Making Framework, grounded in the IFPMA Ethos or value system, to help companies make decisions that balance business objectives and ethical considerations to meet patient needs and the expectations of the medical community, regulators, and society.
Read more
February 2024: Impact of a waiver of intellectual property rights for COVID-19 therapeutics
As discussions on an extension of a waiver of intellectual property (IP) rights on COVID-19 therapeutics continue, latest evidence and data published today explains what the adverse impact of a waiver may be on the entire innovation ecosystem and the consequences it may have on industry’s ability to fight future pandemics.
Read more


