Report
12 Dec 2025
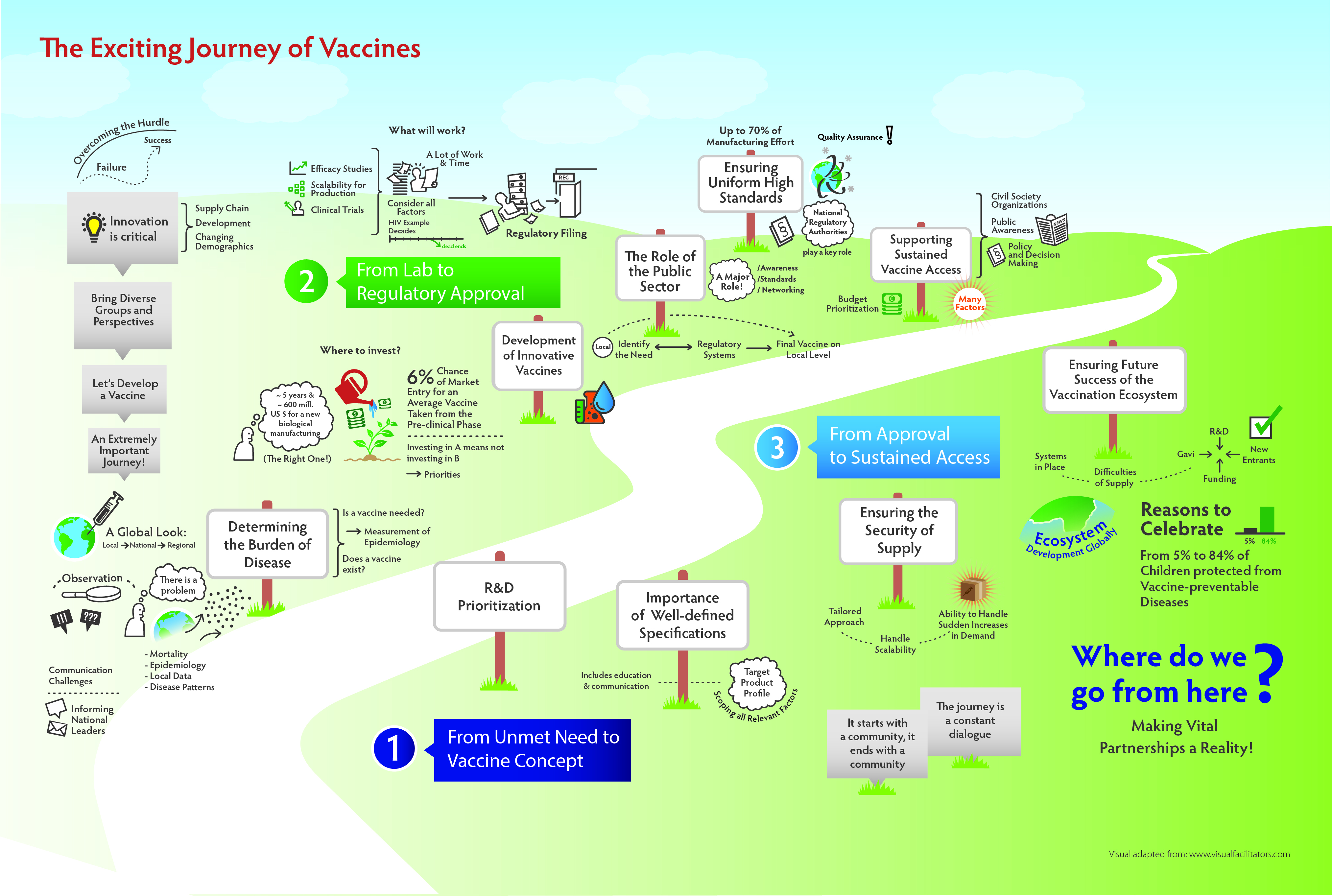
The innovation development and access pathway: A holistic approach to accelerating access to innovative medicines and vaccines
IFPMA introduces the “Innovation Development and Access Pathway” (IDAP), a framework designed to build a shared understanding of the end-to-end pathway of how medicines and vaccines move from discovery to delivery at the point of care. The paper maps the four critical pillars of this journey: R&D and the innovation ecosystem; regulatory processes and life cycle management; listing, procurement and reimbursement; and health service delivery. It identifies systemic challenges, and proposes collaborative solutions to overcome them, and shows that improving access requires coordinated action across stakeholders, with governments playing a central role in enabling timely and equitable access to innovation.
Read more
Position paper
11 Dec 2025
The role of assessment reports in enabling regulatory reliance – perspectives from the pharmaceutical industry
Reliance in decision-making offers a pathway for national regulatory authorities (NRAs) to consider evaluations conducted by other NRAs while maintaining accountability for their decisions. By avoiding duplicative reviews and optimizing resource allocation, the use of regulatory reliance can lead to faster patient access to safe, effective, and high-quality medical products in a timely manner. A...
Read more
Position paper
11 Dec 2025
Best practices for unilateral reliance for initial marketing authorization and post-approval changes
Regulatory reliance has emerged as a key strategy for strengthening regulatory systems by optimizing resources, reducing duplication, and accelerating access to high-quality medicines. By leveraging the expertise and prior decisions of trusted national regulatory authorities (NRAs), reliance allows NRAs to focus their efforts on critical public health priorities while maintaining sovereignty over final regulatory decisions....
Read more