Upholding ethics and business integrity


IFPMA leads the way when it comes to maintaining and raising the ethical standards of the innovative pharmaceutical industry. These standards are the industry’s promise of trust, the most essential ingredient in innovation and patient welfare.
Overview
We carry out a vital role in providing guidance to the innovative pharmaceutical industry when it comes to ethical decision-making and preserving business integrity, with patient health and well-being at the core of our mission. All ethics and business integrity-related materials are gathered in our Ethoscope. This framework incorporates our Ethos and our Code of Practice and offers essential tools to guide the work of our members and the wider sector.
Ethoscope: Ethics essentials in one place
IFPMA’s Ethoscope is a framework that puts ethics and business integrity into action. It includes our expectations on ethical standards and offers guidance on diverse topics to provide clarity and promote continued high-standard alignment across our industry.
Our Ethoscope helps members, patients, and other stakeholders in the global health community cooperate ethically and helps our industry build and sustain trust and innovate responsibly.

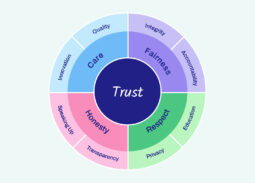
The Ethoscope is driven by our Ethos. The Ethos sets out our industry’s guiding principles, founded on trust and supported by our values of care, fairness, respect, and honesty.
Our Ethos underpins the rules of our Code of Practice. Since 1981, the IFPMA Code has provided our members with global standards for ethical conduct and engagements with key stakeholders.
Over the years, the Code has been updated and Notes for Guidance have been introduced to keep pace with emerging developments in the ever-changing health ecosystem, such as scientific innovations, emerging technologies, and new business models. These require agile guidance on acceptable business practices.
Ethoscope resources
Our Ethos
The Ethos instils a culture of ethics and integrity that guides the way we behave and interact with the rest of the global health community. It is centered on trust, care, fairness, respect, and honesty. Resources include:
- Ethos poster
- Ethos in Action (Decision-Making Framework)

Code of Practice
The Code of Practice governs how IFPMA members operate, communicate, and act in an ethical manner and in line with societal expectations. Resources include:

Additional guidance and best practices
Notes for Guidance are non-binding documents issued by IFPMA providing advice to aid in interpreting the Code of Practice, and/or to address a new topic of concern. They are agile instruments that allow for flexibility in approach. Resources include:
- Sponsorship of events and meetings (2023)
- Social media and digital channels (2022)
- AI ethics principles (2022)
- Diversity and inclusion in clinical trials (2022)
- Virtual and hybrid international medical congresses (2022)
- Data ethics principles (2021)
- Telemedicine (2021)
- Ethical third-party intermediary relationships (2020)
- Fees for services (2020)
- Patient and patient organization interactions (2020)
- Continuing medical education (2018)

New Note for Guidance: Social media and digital channels
The guidance describes principles applicable to communications on social media and digital channels. It helps members identify appropriate information to share across different digital channels while measuring the risk. Also, it describes the general use of different types of digital channels, and provides specific guidelines when engaging with online influencers and digital opinion leaders.

The Ethoscope provides guidance for the innovative pharmaceutical industry, the wider sector, and its stakeholders. For example, to a professional congress organizer or a pharmaceutical company needing to establish the conditions under which they can invite doctors to speak at their event, or what kind of promotional material they can distribute.

The positive return on ethics
Health-related small- and medium-sized enterprises (SMEs) with mature ethics and compliance programs significantly outperformed those with low maturity across key economic performance indicators during the pandemic. This includes revenue growth and international expansion.
Healthcare ecosystem outreach
An ethical healthcare ecosystem can only be achieved together. The innovative pharmaceutical industry is committed to joining with all vital actors, from healthcare professionals and patients to regulators and investors, to realize and preserve a culture of integrity.
These partnerships with stakeholders enable a consistently high standard and aligned approach to ethical business conduct.
Our work with multilateral organizations, including with the Basel Institute on Governance, the Business Ethics for APEC SMEs Initiative, and Consensus Frameworks for Ethical Collaboration, are examples of our healthcare ecosystem outreach.

Basel Institute on Governance
The Basel Institute on Governance is an independent nonprofit headquartered in Basel, Switzerland since 2003 dedicated to countering corruption and other financial crimes and to improving standards of governance.
Read more
Business Ethics for APEC SMEs Initiative
The Business Ethics for APEC SMEs initiative is the world’s largest public-private partnership to strengthen ethical business practices in the innovative pharmaceutical sector.
Read more
Consensus Frameworks for Ethical Collaboration
Consensus Frameworks for Ethical Collaboration are multi-stakeholder, collective action initiatives that outline shared principles and a common approach to guiding ethical conduct and collaboration for actors in healthcare
Read more
CECO Roundtable
The CECO Roundtable is a strategic advisory body, providing a unique global policy platform on the topic of Ethics and Integrity, thus reinforcing IFPMA’s voice on this matter and prioritizing industry engagement.

Take our Code of Practice eLearning course
Our Code of Practice eLearning course is an interactive learning resource designed to help IFPMA members get up to speed on what has changed in the Code, and to consider how the Code can help them make good decisions in their working lives.
Discover our Ethos in Action
Decision-making that integrates business and ethics into day-to-day business activities bolsters patient and public trust, which is essential for innovation and critical interactions with patients, healthcare professionals, healtcare organizations, third parties, peer companies, the industry, and society.