#AlwaysInnovating: The importance of pharmaceutical innovation

Since I joined the pharmaceutical industry over 35 years ago, we have seen incredible scientific progress in how we diagnose, prevent, and treat disease. A new generation of cancer treatments have transformed outcomes for patients. We’ve seen mRNA vaccines developed in response to the COVID pandemic, and personalized medicines have become a reality for patients with rare diseases. This progress has been driven by pharmaceutical innovation, led by companies with deep scientific experience and expertise.
While this progress has been transformational, the world continues to face major health threats. For example, tackling the growing threat of anti-microbial resistance will require significant innovation by pharmaceutical companies, supported by action from countries and health systems around the world.
But new medicines and vaccines don’t fall out of the sky. It is critical that we understand and protect what makes this innovation possible. As an industry, we must get better at telling this story.
But new medicines and vaccines don’t fall out of the sky. It is critical that we understand and protect what makes this innovation possible. As an industry, we must get better at telling this story.
That’s why IFPMA has launched our #AlwaysInnovating campaign, which lifts the curtain on this journey, revealing the expertise, collaboration, and perseverance it takes to turn an idea into a real-world medicine or vaccine that brings solutions to patients in need.
The pharmaceutical innovation journey
The pharmaceutical innovation journey can be long and is often fraught with challenges. But every time we fail, we gain new knowledge to help us move forward on the journey – deepening our understanding of the human body, of different diseases, and how they respond to different medicines.
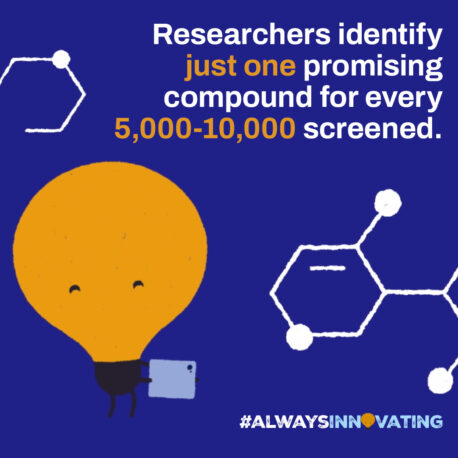
In 2022, a total of 64 novel active substances (NAS) were launched globally, with over 6,147 products in active development from Phase 1 to regulatory submission. Today, there are more than 9,000 medicines in development across all therapeutic fields, and 260 vaccines in the pipeline.
Many won’t make it to the finishing line.
For example, there have been decades of research into therapies for Alzheimer’s, with mostly disappointing results. Now, advances mean that we are finally seeing Alzheimer’s disease modifying treatments further through the innovation journey. While they may not be silver bullets yet, they still offer us hope that we can slowly but surely reduce the symptoms of Alzheimer’s and improve the quality of people’s lives.
Sometimes our industry investigates an idea with a specific disease in mind, and then, through wider exploration, we discover it offers the potential to treat different diseases, like the drug initially tested for Ebola without success that turned out to be effective at treating COVID-19. Similarly, mRNA has been in development for a decade to treat cancer, but it found its first application for COVID-19.
A competitive scientific ecosystem can also help accelerate innovation. Another good example is treatments for Hepatitis C. In 25 years, we have gone from being able to diagnose the disease to treating it – albeit with significant adverse effects – to a cure. Direct-acting antivirals were the game changer, offering a cure, shorter treatment durations, and fewer side effects, and leading to a significant reduction in Hepatitis C-related deaths and hospitalizations.
But innovation is not only discovering a cure or preventative measures for previously untreatable diseases. We are also committed to developing more effective therapies, developing heat-stable formulations that are easier to store and deliver in low-resource settings, child-friendly solutions that facilitate administration, and shorter regimens that patients are more likely to complete. As an example, treatments for tuberculosis are being adapted, enabling shorter, simplified medication regimens to ensure patients can access and adhere to our medicines, going from 24 months to four months of treatment.
Protecting the medical innovations of today and tomorrow
We must protect the innovation ecosystem that makes this journey possible. Because the innovation journey is itself so unpredictable, we need the strong foundations on which to build it.
Having a system that provides a framework to manage the risks, costs, and time involved in pursuing new medicines is central – and the intellectual property (IP) framework is core to this. IP also enables companies to collaborate and share knowledge with trusted partners, scaling up the production of medicines and vaccines, as we have seen during the COVID-19 pandemic, with over 401 manufacturing deals for COVID-19 vaccines, and 158 for COVID-19 therapeutics.
Our new campaign goes behind the scenes of innovation to help more people understand that it’s a long, complex, and risky process, and show how much the industry invests – in terms of ingenuity, time, and resources – to make scientific innovation happen.
With the right policies in place to ensure our innovation ecosystem keeps conquering the ups and downs, we will continue to unlock innovative solutions to some of the world’s most pressing health challenges.
Get the full story behind our #AlwaysInnovating campaign
Pharmaceutical innovation – the core of our industry’s work – relies on pushing the limits of scientific knowledge to advance new medicines and vaccines for the benefit of people everywhere.
#AlwaysInnovating
Author